Our Research Interests
Delving into the intricate world of lipid metabolism, we meticulously dissect how variations in genes and metabolites within this complex framework contribute to the onset and progression of various diseases. A central theme of our laboratory that connects these diverse research areas is lipid droplet biology. These organelles represent the primary storage form of energy in nearly all cell types and their excess in most tissues is a sign of metabolic stress/dysfunction. Our findings to date have led us down numerous research avenues, discussed below, that further explore the biochemistry and cell biology of lipid droplets in a variety of cell types (liver, brain, muscle, adipose) as well as translational studies tightly intertwined with lipid droplet biology. Our laboratory employs a wide range of experimental techniques ranging from basic biochemistry (recombinant protein), cell biology (microscopy, cell culture), to animal models (feeding studies, exercise physiology).
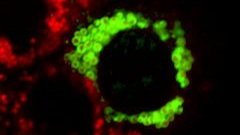
Perilipins and Lipid Droplet Signaling
Lipid droplets (LDs) provide a reservoir for triacylglycerol storage and are a central hub for fatty acid trafficking and signaling in cells. Lipolysis (the break down of fat) promotes mitochondrial biogenesis and oxidative metabolism via a SIRT1/PGC-1α/PPARα-dependent pathway. We identified that monounsaturated fatty acids (MUFAs) allosterically activate SIRT1 toward select peptide-substrates such as PGC-1α. MUFAs enhance PGC-1α/PPARα signaling and promote oxidative metabolism in cells and animal models in a SIRT1-dependent manner. Moreover, we characterized the LD protein perilipin 5 (PLIN5), which is known to enhance mitochondrial biogenesis and function, to be a fatty-acid-binding protein that preferentially binds LD-derived monounsaturated fatty acids and traffics them to the nucleus following cAMP/PKA-mediated lipolytic stimulation. Thus, these studies identify the first-known endogenous allosteric modulators of SIRT1 and characterize a LD-nuclear signaling axis that underlies the known metabolic benefits of MUFAs and PLIN5. We are expanding upon these studies by examining dietary and exercise interventions impact on this pathway. Moreover, how these interventions can be used to promote healthy aging is a major goal of the Najt lab.
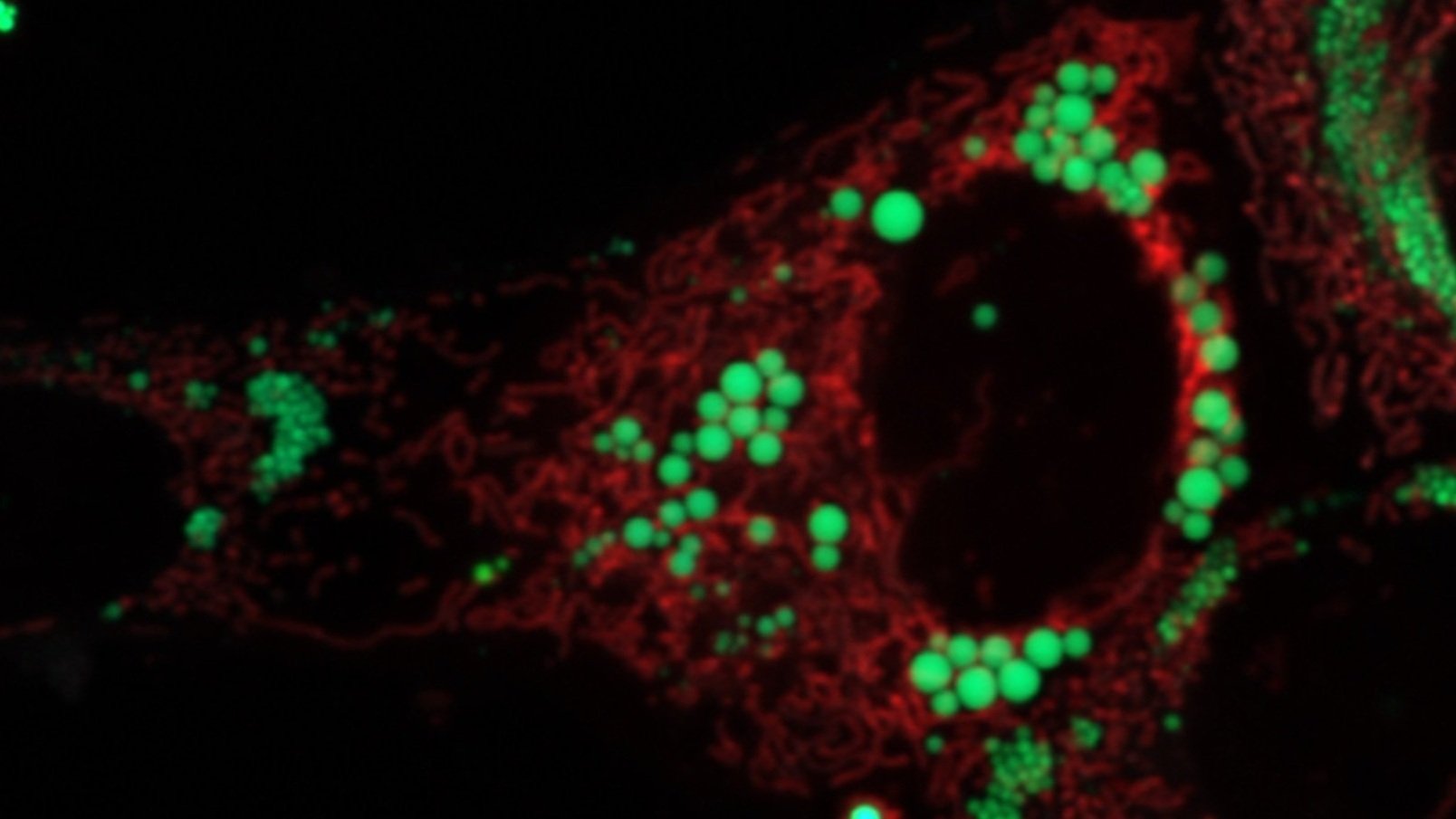
Lipid Droplets in Aging
Interventions such as caloric restriction, fasting regiments (intermittent fasting and time-restricted feeding), or exercise are well known to promote lipid droplet breakdown and, to no surprise, are also known to have very positive and wide-ranging health benefits. Thus, we are very interested in defining the importance of lipid droplet breakdown as a critical mediator of these interventions. In addition, the composition lipid droplets appears to be a key factor dictating lipid droplet signaling. As a result, we are very interested in determining how different dietary fats, which alter lipid droplet composition, interact with fasting to influence disease development and the aging process. The focus of the Najt lab under this research area is focused on the nuclear-LD-mitochondiral signaling access the PLIN5 coordinates. By minipulating PLIN5 mutliple functions, we expected to link metabolic sensing to adaptations associated with increased health and longevity.
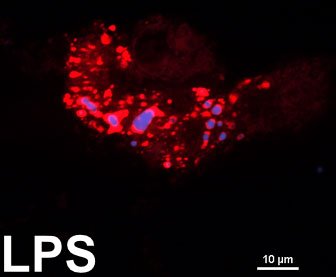
Lipid Droplets and Inflammation
Numerous studies have linked lipid droplet accumulation to inflammation. Since chronic inflammation underlies most diseases, we are keen on understanding the detailed mechanisms through which changes in the protein or lipid composition of lipid droplets regulate inflammation. In particular, we are identifying and characterizing the proteins that couple or uncouple lipid droplets from inflammation. The vast majority of the work done by the Najt lab in this area is focused on perilipin 2 (PLIN2). PLIN2 is often thought to be a blocker of lipolysis, however we have recently shown that PLIN2 is pro-inflammatory. We are investigating the mechanisms by which PLIN2 switchs from a metabolic regulator to a pro-inflammatory protein. In addition, numerous studies are ongoing to define how lipid droplet-mediated regulation of inflammation drives the development of various metabolic diseases and contributes to the aging process. An area that the Najt lab is expanding into is that of inflammation and the brain!
